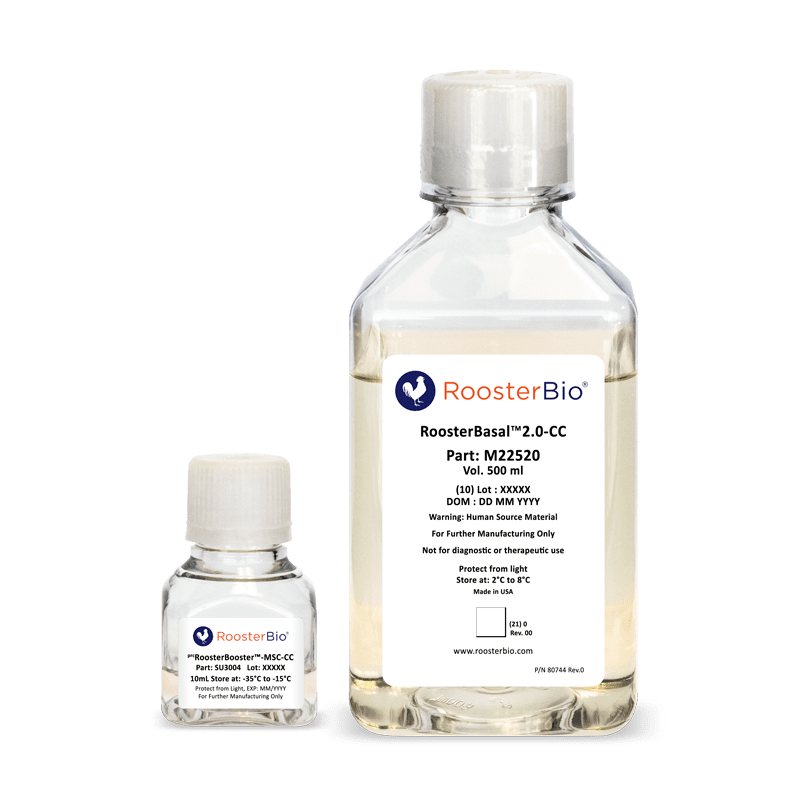
prcRoosterNourish™-MSC-CC
A cGMP bioprocess medium with pathogen reduced components for therapeutic hMSC manufacturing.
Designed for allogeneic and autologous hMSC manufacturing processes.
Recommended Products
Overview
prcRoosterNourish™-MSC-CC is RoosterBio’s xeno-free (XF), GMP bioprocess medium designed to eliminate media exchanges during rapid expansion of human MSCs for clinical allogeneic and autologous therapeutic cell manufacturing. Formulated utilizing irradiated ‘pathogen reduced components’ (prc), this medium provides an enhanced regulatory profile to meet evolving global guidances.
This medium supports in vitro expansion of hMSCs in a 2D batch process or in a 3D bioreactor fed-batch process with our prcRoosterReplenish™-MSC-CC bioreactor feed, achieving unparalleled productivity (Millions of cells produced per Liters of media consumed) compared to other MSC media on the market. Used alongside our CC RoosterVial™-MSC and following RoosterBio® protocols, this system generates the billions of cells necessary to meet clinical needs.
CliniControl™ bioprocess media are manufactured with methods and controls that conform with current Good Manufacturing Practices (cGMP) and are supported for use as an ancillary material by a Type II Master File on file with the FDA. Time spent on investigational new drug (IND) submissions is significantly reduced by facilitating CMC documentation.
Product Features
- Xeno-free | cGMP | Pathogen Reduced (prc)*
- No media exchanges required
- hMSC expansion media for batch and fed-batch (when using prcRoosterReplenish-MSC-CC) culture systems
- No surface coatings required when using Corning CellBind™ surfaces
- (K82304) 500 ml bottle RoosterBasal™2.0-CC with 10 mL bottle of prcRoosterBooster™-MSC-CC
- Also available in 10L bagged RoosterBasal™2.0-CC with 2x 100 mL bags of prcRoosterBooster™-MSC-CC
- Available in custom formats
- Supported by US FDA Type II Master Files – available for cross reference
Intended Use: For Further Manufacturing Use Only. Not intended for diagnostic use or as an excipient for direct human or veterinary therapeutic use.
*’prc’ denoted products have been manufactured using pathogen reduced components to meet evolving regulatory needs and standards.
Protocols and Information
Login to search Certificates of Analysis and Quality Control Briefs.
Scientific Resources
Blog Articles
Publications
An Effective Peptide-Based Platform for Efficient Exosomal Loading & Cellular Delivery of a MicroRNA

Expansion & Characterization of Human Limbus-derived Stromal/mesenchymal Stem Cells in Xeno-free Medium for Therapeutic Applications

Upscaling Human Mesenchymal Stromal Cell Production in a Novel Vertical-wheel Bioreactor Enhances Extracellular Vesicle Secretion & Cargo Profile

Refrigerated Human Mesenchymal Stromal Cells as an Alternative to Cryostorage for Use in Clinical Investigation

Tailoring the Secretome Composition of Mesenchymal Stem Cells to Augment Specific Functions of Epidermal Regeneration: an in vitro Diabetic Model

Comparison of the Therapeutic Effects Between Stem Cells & Exosomes in Primary Ovarian Insufficiency: as Promising as Cells But Different Persistency & Dosage

Characterizing on‐Chip Angiogenesis Induction in a Microphysiological System as a Functional Measure of Mesenchymal Stromal Cell Bioactivity

Inflammatory Licensed hMSCs Exhibit Enhanced Immunomodulatory Capacity in a Biomaterial Mediated Manner

High-Throughput Bioprinting of Geometrically-Controlled Pre-Vascularized Injectable Microgels for Accelerated Tissue Regeneration

Umbilical Cord Stem Cell Lysate: a New Biologic Injectate for the Putative Treatment of Acute Temporomandibular Joint Inflammation

Mesenchymal Stem Cell Extracellular Vesicles from Tissue-Mimetic System Enhance Epidermal Regeneration Via Formation of Migratory Cell Sheets

Evaluating Osteogenic Effects Associated With the Incorporation of Ascorbic Acid in Mineralized Collagen Scaffolds
Human Macrophage Migration Inhibitory Factor Potentiates Mesenchymal Stromal Cell Efficacy in a Clinically Relevant Model of Allergic Asthma