“One morning I woke up… A new day, a new way… And new eyes to see the dawn.” [1]
Ah, the joy of discovery, like Promethean fire wrested from the Cosmos! You can imagine a similar mood of boundless possibility steeped in the drafting of Dr. Arnold Caplan’s landmark 1991 publication, Mesenchymal Stem Cells. [2] Perhaps Milstein and Köhler could almost taste this kind of euphoria when monoclonal antibodies (mAbs) were first generated by 1975 [3]?
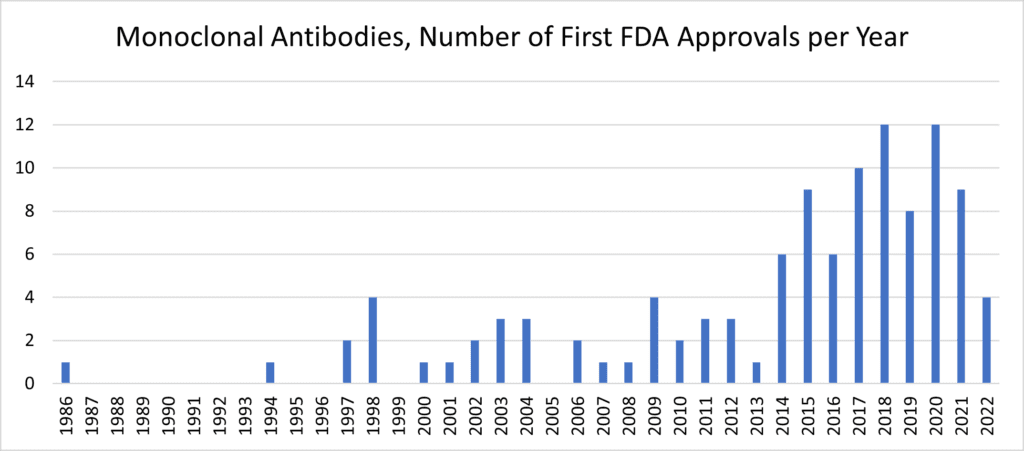
Following the 30-year template, we’d expect mesenchymal stromal/stem cells (MSCs) to similarly boom in the 2020s after their initial characterization in the 1990s. Therapeutic exosomes and EVs—many sourced from MSC producer cells—would likewise be on track to make huge post-clinical advances later in this decade. [13] Yet it behooves us to ask “why?” Despite approximately 10 approved MSC products marketed worldwide beyond the USA, [14, 15] why—after such compelling pre-clinical and human safety data—aren’t things progressing more quickly? The simple answer (and I don’t mean to be cheeky) is that it’s complicated. [16] Like other kinds of biotherapeutics, unexpected product development questions generate substantial perspiration to contend with many MSC therapeutics’ first inspirations. [17, 18]
These questions first stem from basic MSC biology—and how to best harness these cells’ innate strengths. This blog will attempt to briefly skim the surface of these perplexing questions, and how we might best answer them. But first, let’s review…
What We Understand
* We know where MSCs come from. [19, 20, 21, 22] During early embryogenesis, MSCs largely originate from mobile angioblasts via the lateral plate mesoderm (LPM), although some might be of neural crest [23] origin. A fraction of these highly plastic multi-fate cells, in turn, settle down to nest themselves around the developing microvasculature, becoming perivascular cells, i.e., pericytes. These cells persist in abundance in vascularized tissues like bone marrow, adipose tissue, umbilical cord, uterine lining, and dental pulp. Because they have the same canonical markers as MSCs, [24] most KOLs consider that many (though not all) of these pericytes are bona fide MSCs.
* We know what MSCs do in the body, in their natural context. [25] In the post-natal human, injury that disrupts blood or lymphatic vessel integrity will trigger pericytes/MSCs to proliferate, [26] recruit phagocytes, [27] and stimulate angiogenesis. [28] MSCs then help to “plug in” the holes of punctured vessels, perhaps differentiating into (and/or recruiting) endothelial or smooth myoblast cells. [29] In the context of a local inflammatory milieu, MSCs cordon the “fire” of cytokines and toxins secreted by immune cells, [30] secrete anti-bacterial and anti-viral polypeptides, [31] and may even donate their mitochondria “batteries” via tunneling nanotubes (TNTs) [32] to the “hazmat crew” of exhausted, debris-clearing macrophages. [33, 34] In this way, MSCs and their secretomes also help prepare a site of injury for tissue restoration and healing.
* We know that MSCs can perform as cures in model cellular therapies. [35] When administered properly, MSCs can reap dramatic results in small and large animal models—and there are numerous apparent signs of efficacy in human trials. [36, 37, 38] Over 1500 of such studies have been posted to the world’s clinical trials databases since 2011, [39] with indications ranging from COVID-19/ARDS to graft-versus-host-disease (GvHD) to stroke. About ten MSC products are now marketed with approvals outside the USA. [14, 15]
Yet, there would be a larger number of approved MSC products if regenerative medicine knew how to better grapple with some basic questions, i.e., “known unknowns.” [17]
What We Don’t Fully Understand (Yet)
* We don’t know why MSCs (prepared and administered mostly the same) perform differently against different disease indications. Could application of precision medicine principles find the right cell-drug for the right patients? [40] This question might pursue answers in the kind of crosstalk between MSCs and their dynamic in vivo environment, [41] post-administration. For example, hypoxia primes MSCs to secrete more VEGF and angiogenic factors, [42, 43] and inflammation with TNF-a or IFN-g triggers expression of immunomodulatory activities like Indoleamine 2,3 Dioxygenase (IDO). [44, 45]
One relevant example involves two IV-injected allogeneic bone-marrow derived MSC product formulations (rexlemestrocel-L and remestemcel-L) against a large assortment of indications since 2005. Of these, only four indications are prioritized for today’s Phase III studies: (1) COVID-19/ARDS, (2) advanced chronic heart failure, (3) GVHD, and (4) chronic lower back pain. Since the green light for Phase III studies demands prerequisite positive data from the Phase IIs, the shift in focus away from earlier pursuits is likely informed by commercial/strategic but also physiologic rationale. Obviously, retrospective mining of patient samples and accompanying primary and secondary endpoint data can provide invaluable new insights. [46] An exercise in keen “respice, adspice, prospice” can support design of new trials with more sophisticated readouts and stratification that might be better tuned in on the patients.
Even with a standard prep of MSCs, injected via the same route, it’s been reported that results can and will vary. One example is when compelling results were observed with large animal models of ARDS [47] and human ex vivo lungs [48] —but similar cell material did not win the primary endpoint in Phase II human ARDS trials. [49] One reason might be that there’s no single presentation of “generic” ARDS; in contrast, perhaps the preclinical models were heavily deterministic across one tightly choreographed disease process. Hence, future MSC trials against ARDS may need to be meticulously stratified into multiple subtypes based on more specific models, these being discrete ARDS diseases. [50]
* We don’t fully understand why MSCs (from different sources and preparations) perform differently against the same disease indication. This begs a question: will MSCs assume different clinically-relevant phenotypes when extracted from different tissues and different donors of different age, expanded to different PDLs (population doubling levels), adhering to different soft or hard substrates, subjected to different shear stresses, fed different media, converted into injectable doses via different formats, and distributed in the body either systemically (as per IV) or locally (e.g., into a joint cavity or wound), under allogeneic or autologous conditions? Obviously, too many published studies to count clearly show that each of these permutations matter (i.e., “nurture” of MSCs being at least as crucial as “nature”). [18, 51]
One exemplar publication [52] compared normal hBM-MSCs with dBM-MSCs from Type II diabetes patients. While both cell sources could exhibit the classic trilineage differentiation, the MSCs from diabetics were far more challenging to establish in culture, with proliferation ability severely reduced. [52, 53] It follows that at the end of a bioprocess with multiple population doublings, some aged or stressed cell sources could perform less like MSCs and more like fibroblasts. [54] Still, other recent studies use cells and conditions to suggest the contrary—that some can retain their growth and immunomodulatory characteristics. [55] Another key example is where culture of MSCs as 3D aggregates or spheroids instead of on 2D flat surfaces enhances their in vivo immunomodulatory function. [56]
Significantly, it’s being investigated that putative drivers of MSC stemness like TWIST1 might offer some predictive potency data—to match a discrete cell source material and cognate bioprocess with its specialized clinical application. [57] Questions remain, however. An industrial MSC expansion process that spans from the first seeding of cellular starting material to the final product doses could introduce changes that add up. That is, it’s been reported that clinically relevant cell expansions introduce cumulative differences in clonal cell kinetics, ramifying into an overgrowth of small (or even single) numbers of clones. [58] That’s massively reduced cell diversity over time. If the phenotypic output of this clonal selection pressure is stochastic, lo and behold: we might have a pretty good explanation of why human studies with MSCs can be variable from patient to patient (e.g., dosed from different production lots).
* We don’t fully understand the mechanisms of action of MSCs that are shot-gunned at the myriad unique disease indications. What makes MSCs unique and thereby “medicinal?” For “thrills and giggles,” I wanted to see for myself—somewhat bias-free? By tweaking a well-known online protein interactome visualization tool [59] I could tease out a signature network for MSCs, using the input of 21 genes that are reportedly upregulated in expression [60] in a comparison of MSCs with mature skin fibroblasts; I also added in HSC70 (Stro-1), CD166, CD106, CD90, and CD44 as classic MSC+ markers as well as TWIST1. Next, I asked this algorithm to fill in the gaps with 5 “first shell” interactors of its own “choosing.” The mapping results (a graphical summary of the interactome’s enrichment in MSC-related publications, below) tell us that we picked a constellation of genes closely related to MSCs and that this panel is heavily involved in biological process (GO:) terms associated with development/morphogenesis, cell adhesion, ECM production, tissue elasticity, modulation of WNT signaling, and downregulation of TGF-beta signals (i.e., Smad7). So, yes… MSCs like to secrete lots of stuff, and may keep close the memory of their prenatal life.
Presumably fibroblasts—which are less inclined to show fate plasticity [61]—have fewer stem-like markers than MSCs [62] and will secrete matrix that’s less prone to elastic recoil. However, it may be that distinctions between fibroblasts and MSCs can be blurry [63] and present a perplexing continuum of intermediate phenotypes. This doesn’t exactly explain how MSCs work, but perhaps future “omics” studies can use more erudite approaches to dissect MSCs’ unique “talents” that set them apart from their cellular “cousins?”
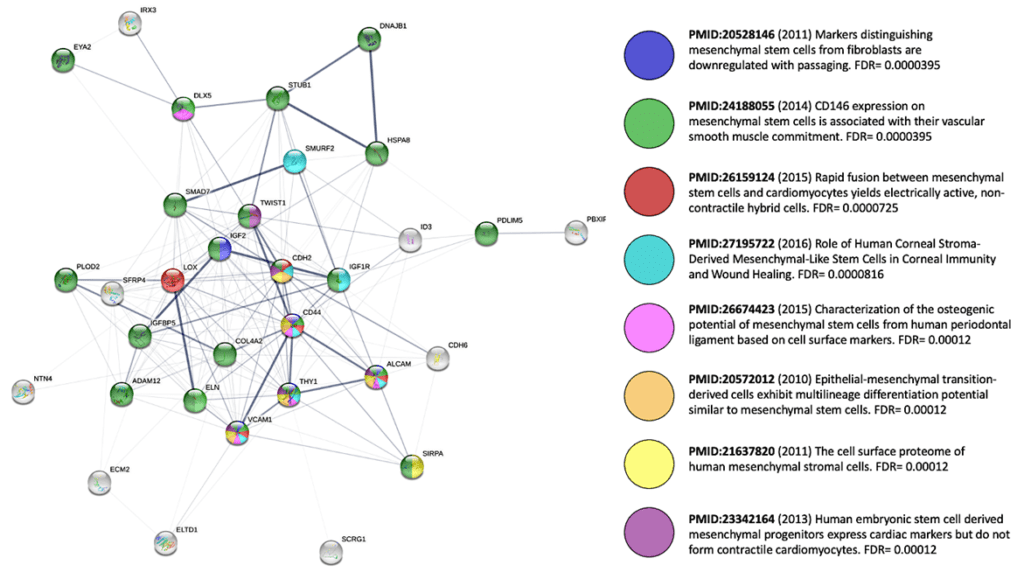
Even if this casual glimpse offers no “slam dunk” answer, further use of the string-db tool [59] or parallel software suites such as Ingenuity or Genomatix [64, 65] could reveal insights we that we might wish to augment therapeutically. If you tweak my aforementioned output by adding 10 additional “first shell” interactors, we can see fascinating KEGG Pathways appear, like hsa0413—Longevity regulating pathway and hsa04066—HIF-1 signaling pathway. Could these carefully annotated pathways be relevant to prospective MSC therapies? Perhaps genetic modification or priming strategies could be aimed at increasing expression of additional positive mediators of such networks to make them more robust, in an indication-selective fashion.
There’s a friendly “debate” at conferences where MSC work is prominent: do MSCs mechanistically benefit humans by (a) direct contact with other cells, or (b), is it what they secrete? (HINT: we all know both happens.) For their part in immunomodulation, the 130-year-old observation of plazentallen [46] offers us what may be an important clue. Pregnant women’s lungs are “dosed” with several grams of fetal stromal cellular materials daily—they circulate from the uterus. Why no anaphylaxis? Apparently, the clearance of fetal cell debris by maternal lung phagocytes may mediate a kind of systemic tolerance of the mother toward her unborn baby. Perhaps the potent effects mediated by MSCs to quiet the immune system “naturally” recapitulate that phenomenon, since IV-injected MSCs are cleared within days after embedding themselves in lung.
If plazentallen is indeed how to explain MSC immune modulation, is it possible that there could be no added benefit to keep MSCs doses alive [66] for such kinds of systemic anti-inflammatory treatments? Maybe so, but this certainly doesn’t mean that MSCs can’t play an important role when injected by alternative routes that confer more long-term viability by orders of magnitude, such as into skeletal muscle. [67] As for the hypothesized role of MSCs to mitigate damage and integrate themselves as regenerative cells, it’s not yet evident that they can do this very well without “help,” i.e., ex vivo tissue engineering in reimplanted scaffolds, encapsulations, and matrices (as expertly reviewed in Levy, et al 2020). [18] Investigation of controlled MSC cell fate thus remains a bedrock of biomedical tissue engineering studies.
MSC’s “secretome” is fertile ground for fast-growing and abundant literature that pertains not only to protein signaling factors (like FGF2, HGF, PDGF, or KGF) or ECM, but also to extracellular vesicles (EVs), of which exosomes are a major sub-class. [68, 69, 70] In many examples, EVs are at least sufficient [71] and possibly necessary [72] for MSCs to contribute a therapeutic effect. The application of MSC-EVs as non-living surrogates of MSC bioactivity [73] to be stockpiled and dosed for off-the-shelf use—without the stringent demands of live cells—could pose a major advantage. However, there’s still debate over what these EVs actually do.
We know EVs/exosomes contribute to cell migration and tissue remodeling through specific kinds of ECM triggered events. They can display (or artificially express) tolerance induction markers like PD-L1, facilitating immunomodulation. [74, 75] They also encapsulate intracellular components from their parent cells and release them for uptake by neighbors, but what’s inside? MSC-EVs contain bioactive miRNAs, cytoplasmic mRNA fragments, protein and lipid signaling complexes, enzyme activities, and possibly even organelles (or alternatively, an organelle-like activity). Are molecular components like miRNAs [76] simply “along for the ride,” [77] or do some mediate meaningful inter-cellular communication? [78] Or perhaps miRNAs (i.e., as “master” post-transcriptional regulators in the producer cell) yield therapeutic EVs via a secondary effect? Like MSC types, there’s considerable heterogeneity in exosomes and EVs, and so it remains challenging to decipher what’s truly relevant towards a therapeutic effect, whether at the level of whole subpopulations or their contents. Improved artificial engineering of EVs can of course render them more druggable, an area of active investigation. [79]
Standard Analytics: The Way Forward…
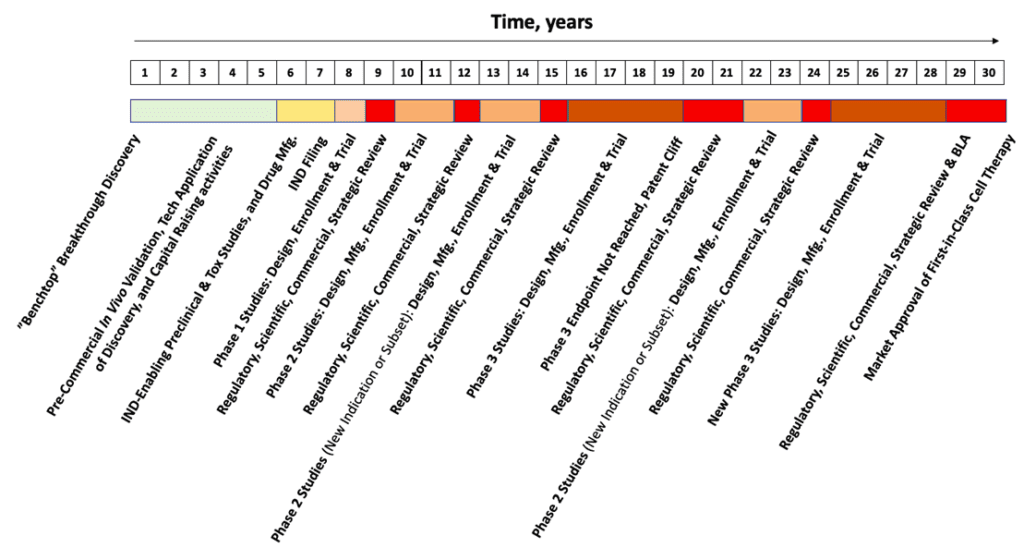
The duration of each individual segment of the Hypothetical Biotech Timeline (above, HBT) is nothing absurd when compared with the launches from other cutting-edge, therapeutic biotech platforms. Yet, taken together, 30 years is obviously a long time. Like an itch that cannot be scratched, that’s too long of a wait, especially for patients in need. Why? The reason is the complexity of the therapy. From this complexity comes inevitable questions that materialize out diverse pools of specialized knowledge at each critical decision point. This is no cause to torpedo one’s optimism, but perhaps instead to temper it with a broader view of where and how success might come about?
As an industry, for MSCs approaching their “30-year itch,” all aspects of this development timeline can (and will) be greatly shortened by applying few basic principles. The key is standard analytics aimed at consistent validation of each critical quality attribute (CQA) of the cell-drug product—leveraged across multiple batches—for decades of bioprocess across the product life cycle. Of course, standards go hand-in-hand with analytics to enforce quality control across necessary modifications in production scheme during scale-up. Some imperatives that might propel the flotilla of rigorous, standardized, testing and analytics are as follows:
- Standardize the MSC preparations [80] according to -omics and stable phenotypes, and apply a matrix of these cell materials against an array of standard model disease processes. [81, 82] A prerequisite for these MSCs is a standardized and industrialized supply chain of off-the-shelf reagents and tools. Such efforts are being kick-started by National Institute for Innovation in Manufacturing Biopharmaceuticals (NIIMBL), the National Institute of Standards and Technology (NIST), the Alliance for Regenerative Medicine (ARM), the National Science Foundation Engineering Research Center for Cell Manufacturing Technologies (CMaT), and the Advanced Regenerative Manufacturing Institute (ARMI) and others, in cooperation with leading industry KOLs. [83] Standardized and precise genetic disease models, [84] using utilizing organs-on-a-chip and large animal models, [85, 86, 87] can dovetail with the most reliable cellular therapy preparations [88] to yield actionable precision medicine insights. [40]
- Radically shorten the timeline [89] to produce a manufacturable batch of cGMP patient doses. [90] Minimizing technician hands-on time via automation and/or high-efficiency media, and simplification of adherent cells’ downstream processing at “right scale” not only reduces waste, [91] but cost as well. [92] By easy access to regulatory expertise and starting and ancillary materials with pre-existing drug master files (DMFs), an MSC product developer can already have complete ~70% of their CMC section by simply cross-referencing the DMF. [93] This can also shave multiple years off the product timeline—and many millions of dollars.
- Recover samples and data from prior trials’ biopsies to formulate more robust hypotheses [94] informed by next-gen, multi-omics precision or personalized medicine [95] As computational power drives the decades-long, logarithmic increase in pattern recognition and data-processing speed, [96, 97] the resulting AI algorithms will turn “dusty,” old samples into a goldmine for new insights. The molecular phenotypes of the MSCs are the “inputs”, [98] and their readouts via patient blood or tissues and their trial outcomes are the “outputs.” Between input and output, the mysterious knowledge gap in the middle will not be a “black box” for much longer.
- Prime and/or genetically engineer MSCs to express potent therapeutic transgenes. Not only would these control levers “supercharge” MSCs à la the CAR-T therapeutic paradigm, but [99] they could also override much of the genetic drift that may occur during cell manufacture and processing. That is, we can transform MSCs into in vivo, living controlled delivery systems [100] for genetic medicines [101] instead of using as merely the single active [102]
- Integrate a standard genetic module system into MSCs for plug-and-play molecular theranostics. This would enable each individual MSC to report on the spatiotemporal or pathological conditions within its environment [103, 104] and allow this cell therapy population to be tracked and controlled, non-invasively, in real time. That’s because the diagnostic controller’s molecular circuitry is functionally linked to its cognate therapeutic effector. Obviously, such would require a very interdisciplinary set of skills to come together under one roof, but the high-quality data and novel therapeutic cures would be worth it!
These are just a few thoughts. And yet, surely, I’m missing something. What do you think?
“Carry On”
Variants of the Chinese hamster ovary (CHO) cell were soon adopted as the workhorse for mAb production. The product from these was determined to be better folded and glycosylated than mAbs from bacteria or yeast, and more amenable to scale up than via hybridomas. Once CHO became the standard for mAb products, the optimized materials and methods for stable clone selection, favorable nutrient flux, bioreactor expansion, and high-density product secretion needed several rounds of process innovation. Given curveballs thrown by mother nature, assays and protocols that didn’t exist previously were met by new analytical methods. These answered the germane questions in due time—empowering more potent, versatile, and manufacturable mAbs for launch on their path to commercialization. [105]
Like mAbs and gene therapies before them, MSCs have also become something of a regenmed standard, [106] a “workhorse” that delivered initial triumphs and ongoing cause for hope. Yet the work is far from over. Accordingly, RoosterBio is answering our customers’ needs to provide a full set of analytics services for MSCs [107] and exosomes/EVs. [108] This will help to optimally match a cell or exosome/EV phenotype with the desired research, pre-clinical or clinical use case. Yes, there are questions that need answering, but that’s why we’re here. “We have no choice but to Carry on!” [1]
Stay tuned for an upcoming blog series on these critical analytics as they relate to MSCs and EVs/exosomes.
References
- Crosby, Stills & Nash. Carry On / Questions. 1970; Available from: https://www.youtube.com/watch?v=nP0VBB7BO64.
- Caplan, A. I., Mesenchymal stem cells. J Orthop Res, 1991. 9(5): p. 641-50. 10.1002/jor.1100090504
- Leavy, Olive, The birth of monoclonal antibodies. Nature Immunology, 2016. 17(1): p. S13-S13.
- Society, The Antibody. Antibody therapeutics approved or in regulatory review in the EU or US. 2022; Available from: https://www.antibodysociety.org/resources/approved-antibodies/.
- Dealmakers, Biopharma, Moving up with the monoclonals. Biopharma Deal, 2019.
- Daley, Jim, Gene therapy arrives. Nature, 2019. 576(7785): p. S12-S12.
- Amgen. FDA Approves IMLYGIC™ (Talimogene Laherparepvec) As First Oncolytic Viral Therapy In The US. 2015; Available from: https://www.amgen.com/newsroom/press-releases/2015/10/fda-approves-imlygic-talimogene-laherparepvec-as-first-oncolytic-viral-therapy-in-the-us.
- Tabara, H., A. Grishok, and C. C. Mello, RNAi in C. elegans: soaking in the genome sequence. Science, 1998. 282(5388): p. 430-1. 10.1126/science.282.5388.430
- Paul, C. P., et al., Effective expression of small interfering RNA in human cells. Nat Biotechnol, 2002. 20(5): p. 505-8. 10.1038/nbt0502-505
- Bumcrot, D., et al., RNAi therapeutics: a potential new class of pharmaceutical drugs. Nat Chem Biol, 2006. 2(12): p. 711-9. 10.1038/nchembio839
- Mullard, Asher, FDA approves landmark RNAi drug. Nature Reviews Drug Discovery, 2018. 17(9): p. 613-613. 10.1038/nrd.2018.152
- Saw, P. E. and E. W. Song, siRNA therapeutics: a clinical reality. Sci China Life Sci, 2020. 63(4): p. 485-500. 10.1007/s11427-018-9438-y
- Zitvogel, L., et al., Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med, 1998. 4(5): p. 594-600. 10.1038/nm0598-594
- Medicine, Alliance for Regenerative. Available Products. 2022; Available from: https://alliancerm.org/available-products/.
- Jossen, V., et al., Manufacturing human mesenchymal stem cells at clinical scale: process and regulatory challenges. Appl Microbiol Biotechnol, 2018. 102(9): p. 3981-3994. 10.1007/s00253-018-8912-x
- Witcher, M. Why Controlling CQAs Isn’t Good Enough For Gene & Cell Therapies. 2020; Available from: https://bit.ly/2xBvCHj.
- Carson, Jonathan. “Known Unknowns” Affect Critical Process Parameters & Critical Quality Attributes in the Bioproduction Cycle. 2021; Available from: https://www.roosterbio.com/blog/known-unknowns-affect-critical-process-parameters-critical-quality-attributes-in-the-bioproduction-cycle/.
- Levy, O., et al., Shattering barriers toward clinically meaningful MSC therapies. Sci Adv, 2020. 6(30): p. eaba6884. 10.1126/sciadv.aba6884
- Crisan, M., et al., A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell, 2008. 3(3): p. 301-13. 10.1016/j.stem.2008.07.003
- Vodyanik, M. A., et al., A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell, 2010. 7(6): p. 718-29. 10.1016/j.stem.2010.11.011
- Slukvin, II and A. Kumar, The mesenchymoangioblast, mesodermal precursor for mesenchymal and endothelial cells. Cell Mol Life Sci, 2018. 75(19): p. 3507-3520. 10.1007/s00018-018-2871-3
- Sheng, G., The developmental basis of mesenchymal stem/stromal cells (MSCs). BMC Dev Biol, 2015. 15: p. 44. 10.1186/s12861-015-0094-5
- Isern, J., et al., The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function. Elife, 2014. 3: p. e03696. 10.7554/eLife.03696
- Caplan, A. I., All MSCs are pericytes? Cell Stem Cell, 2008. 3(3): p. 229-30. 10.1016/j.stem.2008.08.008
- Caplan, A. I. and D. Correa, The MSC: an injury drugstore. Cell Stem Cell, 2011. 9(1): p. 11-5. 10.1016/j.stem.2011.06.008
- Wong, S. P., et al., Pericytes, mesenchymal stem cells and their contributions to tissue repair. Pharmacol Ther, 2015. 151: p. 107-20. 10.1016/j.pharmthera.2015.03.006
- Stark, K., et al., Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat Immunol, 2013. 14(1): p. 41-51. 10.1038/ni.2477
- Watt, S. M., et al., The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. Br Med Bull, 2013. 108: p. 25-53. 10.1093/bmb/ldt031
- Pedersen, T. O., et al., Mesenchymal stem cells induce endothelial cell quiescence and promote capillary formation. Stem Cell Res Ther, 2014. 5(1): p. 23. 10.1186/scrt412
- Bustos, M. L., et al., Activation of human mesenchymal stem cells impacts their therapeutic abilities in lung injury by increasing interleukin (IL)-10 and IL-1RN levels. Stem Cells Transl Med, 2013. 2(11): p. 884-95. 10.5966/sctm.2013-0033
- Sutton, M. T., et al., Antimicrobial Properties of Mesenchymal Stem Cells: Therapeutic Potential for Cystic Fibrosis Infection, and Treatment. Stem Cells Int, 2016. 2016: p. 5303048. 10.1155/2016/5303048
- Soundara Rajan, T., et al., Tunneling Nanotubes-Mediated Protection of Mesenchymal Stem Cells: An Update from Preclinical Studies. Int J Mol Sci, 2020. 21(10). 10.3390/ijms21103481
- Carson, Jonathan. From Old Adversaries to RegenMed Housemates: The 2-Billion-Year Story of Mitochondria Transfer via MSCs. 2021; Available from: https://www.roosterbio.com/blog/from-old-adversaries-to-regenmed-housemates-the-2-billion-year-story-of-mitochondria-transfer-via-mscs/.
- Jackson, M. V., et al., Mitochondrial Transfer via Tunneling Nanotubes is an Important Mechanism by Which Mesenchymal Stem Cells Enhance Macrophage Phagocytosis in the In Vitro and In Vivo Models of ARDS. Stem Cells, 2016. 34(8): p. 2210-23. 10.1002/stem.2372
- Penn, M. S., The Unraveling of the Matryoshka Doll. Circ Res, 2017. 120(7): p. 1075-1077. 10.1161/CIRCRESAHA.116.309904
- Don, W, First off-the-shelf mesenchymal stem cell therapy nears European approval. Nature Biotechnology, 2018. 36(3): p. 213.
- Terry, M, Mesoblast’s Stem Cell Therapy Shows 83% Survival in Ventilator-Dependent COVID-19 Patients. Bio Space Home Page. Available online: https://www.biospace.com/article/mesoblast-ltd-s-stem-cell-therapy-shows-83-percent-survival-in-covid-19-patients/ (accessed on 24 September 2021), 2020.
- Doctor, Vanessa. New Mesoblast Data Offers Hope for High-Risk Heart Patients. 2021; Available from: https://www.biospace.com/article/mesoblast-s-rexlemestrocel-l-demonstrates-greatest-benefit-in-cardio-patients-with-diabetes-myocardial-ischemia/.
- Verter, Frances. MSC Trials 2011-2022. 2022; Available from: https://celltrials.org/public-cells-data/msc-trials-2011-2020/65.
- Carson, Jonathan. MSCs & Precision Medicine: Mining a “Galaxy” of Answers for the “Ultimate” Questions. 2021; Available from: https://www.roosterbio.com/blog/mscs-and-precision-medicine-mining-a-galaxy-of-answers-for-the-ultimate-questions/.
- Greco, S. J. and P. Rameshwar, Microenvironmental considerations in the application of human mesenchymal stem cells in regenerative therapies. Biologics, 2008. 2(4): p. 699-705. 10.2147/btt.s2765
- Crisostomo, P. R., et al., Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am J Physiol Cell Physiol, 2008. 294(3): p. C675-82. 10.1152/ajpcell.00437.2007
- Tamama, K., et al., Differential roles of hypoxia inducible factor subunits in multipotential stromal cells under hypoxic condition. J Cell Biochem, 2011. 112(3): p. 804-17. 10.1002/jcb.22961
- Prasanna, S. J., et al., Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS One, 2010. 5(2): p. e9016. 10.1371/journal.pone.0009016
- Iain Farrance, Priya Baraniak, and Jon Rowley Priming of hMSCs to Improve Potency. 2014; Available from: https://www.roosterbio.com/blog/priming-of-hmscs-to-improve-potency/.
- Galipeau, J. and L. Sensebe, Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell, 2018. 22(6): p. 824-833. 10.1016/j.stem.2018.05.004
- Matthay, M. A., Therapeutic potential of mesenchymal stromal cells for acute respiratory distress syndrome. Ann Am Thorac Soc, 2015. 12 Suppl 1: p. S54-7. 10.1513/AnnalsATS.201406-254MG
- Lee, J. W., et al., Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med, 2013. 187(7): p. 751-60. 10.1164/rccm.201206-0990OC
- Matthay, M. A., et al., Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med, 2019. 7(2): p. 154-162. 10.1016/S2213-2600(18)30418-1
- Calfee, C. S., et al., Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest, 2015. 147(6): p. 1539-1548. 10.1378/chest.14-2454
- O’Connor, K. C., Molecular Profiles of Cell-to-Cell Variation in the Regenerative Potential of Mesenchymal Stromal Cells. Stem Cells Int, 2019. 2019: p. 5924878. 10.1155/2019/5924878
- Phadnis, S. M., et al., Mesenchymal stem cells derived from bone marrow of diabetic patients portrait unique markers influenced by the diabetic microenvironment. Rev Diabet Stud, 2009. 6(4): p. 260-70. 10.1900/RDS.2009.6.260
- Almeria, C., et al., Heterogeneity of mesenchymal stem cell-derived extracellular vesicles is highly impacted by the tissue/cell source and culture conditions. Cell Biosci, 2022. 12(1): p. 51. 10.1186/s13578-022-00786-7
- Halfon, S., et al., Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem Cells Dev, 2011. 20(1): p. 53-66. 10.1089/scd.2010.0040
- Dave, J. R., et al., Human gingival mesenchymal stem cells retain their growth and immunomodulatory characteristics independent of donor age. Sci Adv, 2022. 8(25): p. eabm6504. 10.1126/sciadv.abm6504
- Bartosh, T. J., et al., Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A, 2010. 107(31): p. 13724-9. 10.1073/pnas.1008117107
- Boregowda, S. V., et al., A Clinical Indications Prediction Scale Based on TWIST1 for Human Mesenchymal Stem Cells. EBioMedicine, 2016. 4: p. 62-73. 10.1016/j.ebiom.2015.12.020
- Selich, A., et al., Massive Clonal Selection and Transiently Contributing Clones During Expansion of Mesenchymal Stem Cell Cultures Revealed by Lentiviral RGB-Barcode Technology. Stem Cells Transl Med, 2016. 5(5): p. 591-601. 10.5966/sctm.2015-0176
- Jensen, L. J., et al., STRING 8–a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res, 2009. 37(Database issue): p. D412-6. 10.1093/nar/gkn760
- Brendel, C., et al., Distinct gene expression profile of human mesenchymal stem cells in comparison to skin fibroblasts employing cDNA microarray analysis of 9600 genes. Gene Expr, 2005. 12(4-6): p. 245-57. 10.3727/000000005783992043
- Zupan, Janja, Mesenchymal Stem/Stromal Cells and Fibroblasts: Their Roles in Tissue Injury and Regeneration, and Age-Related Degeneration. Fibroblasts—Advances in Inflammation, Autoimmunity and Cancer, 2021: p. 1-25.
- Pasanisi, E., et al., Differentiation and plasticity of human vascular wall mesenchymal stem cells, dermal fibroblasts and myofibroblasts: a critical comparison including ultrastructural evaluation of osteogenic potential. Ultrastruct Pathol, 2019. 43(6): p. 261-272. 10.1080/01913123.2019.1673863
- Denu, R. A., et al., Fibroblasts and Mesenchymal Stromal/Stem Cells Are Phenotypically Indistinguishable. Acta Haematol, 2016. 136(2): p. 85-97. 10.1159/000445096
- Haydont, V., et al., Fibroblasts from the Human Skin Dermo-Hypodermal Junction are Distinct from Dermal Papillary and Reticular Fibroblasts and from Mesenchymal Stem Cells and Exhibit a Specific Molecular Profile Related to Extracellular Matrix Organization and Modeling. Cells, 2020. 9(2). 10.3390/cells9020368
- Wenuganen, S., et al., Transcriptomics of Long-Term Meditation Practice: Evidence for Prevention or Reversal of Stress Effects Harmful to Health. Medicina (Kaunas), 2021. 57(3). 10.3390/medicina57030218
- Pang, S. H. M., et al., Mesenchymal stromal cell apoptosis is required for their therapeutic function. Nat Commun, 2021. 12(1): p. 6495. 10.1038/s41467-021-26834-3
- Braid, L. R., et al., Intramuscular administration potentiates extended dwell time of mesenchymal stromal cells compared to other routes. Cytotherapy, 2018. 20(2): p. 232-244. 10.1016/j.jcyt.2017.09.013
- Lenzini, Stephen. Big Effects in Small Packages: What Are Extracellular Vesicles, Exosomes, & Microvesicles & Why Are They En Route to the Clinic? 2021; Available from: https://www.roosterbio.com/blog/big-effects-in-small-packages-what-are-extracellular-vesicles-exosomes-microvesicles-why-are-they-en-route-to-the-clinic/.
- Carson, Jonathan. It’s Not Rocket Science – “MSC-EVs” Have the Right Stuff. 2021; Available from: https://www.roosterbio.com/blog/its-not-rocket-science-msc-evs-have-the-right-stuff/.
- Kalluri, R. and V. S. LeBleu, The biology, function, and biomedical applications of exosomes. Science, 2020. 367(6478). 10.1126/science.aau6977
- Bruno, S., et al., Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol, 2009. 20(5): p. 1053-67. 10.1681/ASN.2008070798
- Lee, C., et al., Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation, 2012. 126(22): p. 2601-11. 10.1161/CIRCULATIONAHA.112.114173
- Ha, D. H., et al., Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells, 2020. 9(5). 10.3390/cells9051157
- Yu, B., X. Zhang, and X. Li, Exosomes derived from mesenchymal stem cells. Int J Mol Sci, 2014. 15(3): p. 4142-57. 10.3390/ijms15034142
- Ou, Q., et al., Small extracellular vesicles derived from PD-L1-modified mesenchymal stem cell promote Tregs differentiation and prolong allograft survival. Cell Tissue Res, 2022. 10.1007/s00441-022-03650-9
- Albanese, M., et al., MicroRNAs are minor constituents of extracellular vesicles that are rarely delivered to target cells. PLoS Genet, 2021. 17(12): p. e1009951. 10.1371/journal.pgen.1009951
- Toh, W. S., et al., MSC exosome works through a protein-based mechanism of action. Biochem Soc Trans, 2018. 46(4): p. 843-853. 10.1042/BST20180079
- Meldolesi, J., Exosomes and Ectosomes in Intercellular Communication. Curr Biol, 2018. 28(8): p. R435-R444. 10.1016/j.cub.2018.01.059
- Sutaria, D. S., et al., Achieving the Promise of Therapeutic Extracellular Vesicles: The Devil is in Details of Therapeutic Loading. Pharm Res, 2017. 34(5): p. 1053-1066. 10.1007/s11095-017-2123-5
- Rowley, Jon. Regenerative Medicine Standards Development – an FDA Workshop & an Initiative in Need of Structure. 2014; Available from: https://www.roosterbio.com/blog/regenerative-medicine-standards-development-an-fda-workshop-and-an-initiative-in-need-of-structure/.
- Tissue-engineered disease models. Nat Biomed Eng, 2018. 2(12): p. 879-880. 10.1038/s41551-018-0339-2
- Vernetti, L., et al., Functional Coupling of Human Microphysiology Systems: Intestine, Liver, Kidney Proximal Tubule, Blood-Brain Barrier and Skeletal Muscle. Sci Rep, 2017. 7: p. 42296. 10.1038/srep42296
- Olsen, T. R. and J. A. Rowley, Corporate profile: RoosterBio, Inc. Regen Med, 2018. 13(7): p. 753-757. 10.2217/rme-2018-0092
- Heo, D. N., M. Hospodiuk, and I. T. Ozbolat, Synergistic interplay between human MSCs and HUVECs in 3D spheroids laden in collagen/fibrin hydrogels for bone tissue engineering. Acta Biomater, 2019. 95: p. 348-356. 10.1016/j.actbio.2019.02.046
- Skardal, A., et al., Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci Rep, 2017. 7(1): p. 8837. 10.1038/s41598-017-08879-x
- Echigoya, Y., et al., A Dystrophin Exon-52 Deleted Miniature Pig Model of Duchenne Muscular Dystrophy and Evaluation of Exon Skipping. Int J Mol Sci, 2021. 22(23). 10.3390/ijms222313065
- Cooney, A. L., et al., Lentiviral-mediated phenotypic correction of cystic fibrosis pigs. JCI Insight, 2016. 1(14). 10.1172/jci.insight.88730
- RoosterBio. RoosterBio Inc and the US Army Institute of Surgical Research (USAISR) sign a CRADA to accelerate development of Regenerative Medicine. 2016; Available from: http://www.prweb.com/releases/2016/07/prweb13574551.htm.
- RoosterBio. CliniControl Products Compress Development Timelines for Cell-Based Therapeutics. 2022; Available from: https://www.roosterbio.com/wp-content/uploads/2019/10/Rbio_CliniControl_Brochure_FIN-_-SEP-2019_Top_Bottom_-LRG-file.pdf.
- Consortium, National Cell Manufacturing. Cell Manufacturing Roadmap to 2030. 2019; Available from: https://cellmanufacturingusa.org/sites/default/files/Cell-Manufacturing-Roadmap-to-2030_ForWeb_110819.pdf.
- Omokhowa Agbojo, Maya Lim, and Josephine Lembong Environmental Analysis of Therapeutic hMSC Manufacturing: A Comparison of Multiple Bioprocess Systems. 2021; Available from: https://www.roosterbio.com/blog/environmental-analysis-of-therapeutic-hmsc-manufacturing-a-comparison-of-multiple-bioprocess-systems/.
- Burrows, Andrew. Manufacturing stem cells with RoosterBio founder Jon Rowley. 2019; Available from: https://informaconnect.com/roosterbio-manufacturing-stem-cells-jon-rowley/.
- David Alvaro, Tim Kelly. Moving Beyond the Industrialization of MSCs. 2022; Available from: https://www.pharmasalmanac.com/articles/moving-beyond-the-industrialization-of-mscs.
- Rieger, A. C., et al., Genetic determinants of responsiveness to mesenchymal stem cell injections in non-ischemic dilated cardiomyopathy. EBioMedicine, 2019. 48: p. 377-385. 10.1016/j.ebiom.2019.09.043
- Patel, S. A., et al., Personalizing Stem Cell Research and Therapy: The Arduous Road Ahead or Missed Opportunity? Curr Pharmacogenomics Person Med, 2010. 8(1): p. 25-36. 10.2174/1875692111008010025
- Gibney, Elizabeth, Self-taught AI is best yet at strategy game Go. Nature, 2017. 10(1): p. 68-74.
- Service, R. F., ‘The game has changed.’ AI triumphs at protein folding. Science, 2020. 370(6521): p. 1144-1145. 10.1126/science.370.6521.1144
- Islam, D., et al., Identification and Modulation of Microenvironment Is Crucial for Effective Mesenchymal Stromal Cell Therapy in Acute Lung Injury. Am J Respir Crit Care Med, 2019. 199(10): p. 1214-1224. 10.1164/rccm.201802-0356OC
- Kojima, R., L. Scheller, and M. Fussenegger, Nonimmune cells equipped with T-cell-receptor-like signaling for cancer cell ablation. Nat Chem Biol, 2018. 14(1): p. 42-49. 10.1038/nchembio.2498
- Langer, R., Drug delivery and targeting. Nature, 1998. 392(6679 Suppl): p. 5-10.
- Sharei, A., et al., Ex vivo cytosolic delivery of functional macromolecules to immune cells. PLoS One, 2015. 10(4): p. e0118803. 10.1371/journal.pone.0118803
- RoosterBio. eBook: Genome Editing of MSCs. 2021; Available from: https://www.roosterbio.com/blog/ebook-genome-editing-of-mscs/.
- Nouri, F. S., X. Wang, and A. Hatefi, Genetically engineered theranostic mesenchymal stem cells for the evaluation of the anticancer efficacy of enzyme/prodrug systems. J Control Release, 2015. 200: p. 179-87. 10.1016/j.jconrel.2015.01.003
- Siska, E. K., et al., Generation of an immortalized mesenchymal stem cell line producing a secreted biosensor protein for glucose monitoring. PLoS One, 2017. 12(9): p. e0185498. 10.1371/journal.pone.0185498
- Liu, J. K., The history of monoclonal antibody development – Progress, remaining challenges and future innovations. Ann Med Surg (Lond), 2014. 3(4): p. 113-6. 10.1016/j.amsu.2014.09.001
- Snyder, Jessica. The hMSC Standard in the Ivory Tower. 2021; Available from: https://www.roosterbio.com/blog/the-hmsc-standard-in-the-ivory-tower/.
- RoosterBio. hMSC Analytical Services. 2022; Available from: https://www.roosterbio.com/hmsc-analytical-services/.
- RoosterBio. EV / Exosome Analytical Services. 2022; Available from: https://www.roosterbio.com/ev-exosome-analytical-services/
