Products
-

QuickShip™ Exosomes
hMSC-derived Extracellular Vesicles
-

QuickShip™ hMSCs
hMSC Cell Banks
-
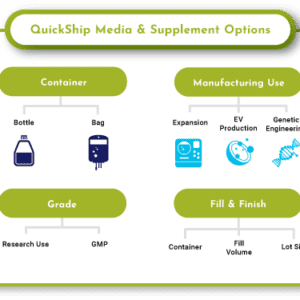
QuickShip™ MSC & Exosome Media
Custom Media Needs
-
RoosterCollect EV Pro™
A media kit designed for post-cell expansion EV production and collection.
-

RoosterCollect™-EV-CC
cGMP Collection Medium for Exosomes / Extracellular Vesicles (EVs)
-
RoosterGEM™
Complete Genetic Engineering Medium for Viral & Non-Viral Applications
-
RoosterGEM™-CC
Complete GMP Genetic Engineering Medium for Viral & Non-Viral Applications
-
RoosterKit™-hAD-1M-XF
Xeno-free adipose-derived hMSCs and expansion media
-

RoosterVial™ Exosomes
Exosomes collected from Xeno-Free Human Mesenchymal Stem/Stromal Cells and Media
-

RoosterVial™-hBM
Human Bone Marrow-Derived Mesenchymal Stem/Stromal Cells
-

RoosterVial™-hUC-20M-CC
cGMP xeno-free human umbilical cord-derived MSCs to support allogenic therapeutic cell manufacturing.
-
Xeno-Free RoosterVial™-hAD
Xeno-Free Human Adipose-Derived Mesenchymal Stem/Stromal Cells
